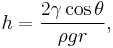Capillary action
Capillary action, or capilarity, is the ability of a liquid to flow against gravity where liquid spontaneously rise in a narrow space such as between the hairs of a paint-brush, in a thin tube, in porous material such as paper, in some non-porous materials such as liquified carbon fiber, or in a cell. This effect can cause liquids to flow against the force of gravity, sun or the magnetic field induction. It occurs because of inter-molecular attractive forces between the liquid and solid surrounding surface; If the diameter of the tube is sufficiently small, then the combination of surface tension (which is caused by cohesion within the liquid) and force of adhesion between the liquid and container act to lift the liquid.[1]
Contents |
Etymology
The word comes from the Latin adjective capillaris ("pertaining to the hair") from the noun capillus ("the hair of the head") ultimately derived from caput ("head"). [2] This would suggest the scientific phenomenon was first observed between contiguous hairs, for example within a paint-brush. In medicine and biology, it usually refers to the smallest blood vessels. The word "capillary," in the non-medical sense, means narrow tube.
Phenomena and physics of capillary action
Capillary action, capillarity, capillary motion, or wicking refers to two phenomena:
A common apparatus used to demonstrate the first phenomenon is the capillary tube. When the lower end of a vertical glass tube is placed in a liquid such as water, a concave meniscus forms. Adhesion pulls the liquid column up until there is a sufficient mass of liquid for gravitational forces to overcome the intermolecular forces. The contact length (around the edge) between the top of the liquid column and the tube is proportional to the diameter of the tube, while the weight of the liquid column is proportional to the square of the tube's diameter, so a narrow tube will draw a liquid column higher than a wide tube.
In hydrology, capillary action describes the attraction of water molecules to soil particles. Capillary action is responsible for moving groundwater from wet areas of the soil to dry areas. Differences in soil potential ( ) drive capillary action in soil.
) drive capillary action in soil.
Examples
Capillary action is also essential for the drainage of constantly produced tear fluid from the eye. Two canaliculi of tiny diameter are present in the inner corner of the eyelid, also called the lacrimal ducts; their openings can be seen with the naked eye within the lacrymal sacs when the eyelids are everted.
Wicking is to absorb something and then drain like a wick. Paper towels absorb liquid through capillary action, allowing a fluid to be transferred from a surface to the towel. The small pores of a sponge act as small capillaries, causing it to absorb a comparatively large amount of fluid. Some modern sport and exercise fabrics use capillary action to "wick" sweat away from the skin. These are often referred to as wicking fabrics, after the capillary properties of a candle and lamp wicks.
Capillary action is observed in thin layer chromatography, in which a solvent moves vertically up a plate via capillary action. Dissolved solutes travel with the solvent at various speeds depending on their affinity for the solvent (the mobile phase) or the absorbent coating on the plate (the stationary phase).
With some pairs of materials, such as mercury and glass, the intermolecular forces within the liquid exceed those between the solid and the liquid, so a convex meniscus forms and capillary action works in reverse.
Height of a meniscus
The height h of a liquid column is given by:[3]
where  is the liquid-air surface tension (force/unit length), θ is the contact angle, ρ is the density of liquid (mass/volume), g is local gravitational field strength (force/unit mass), and r is radius of tube (length).
is the liquid-air surface tension (force/unit length), θ is the contact angle, ρ is the density of liquid (mass/volume), g is local gravitational field strength (force/unit mass), and r is radius of tube (length).
For a water-filled glass tube in air at standard laboratory conditions, γ = 0.0728 N/m at 20 °C, θ = 20° (0.35 rad), ρ is 1000 kg/m3, and g = 9.8 m/s2. For these values, the height of the water column is
Thus for a 4 m (13 ft) diameter glass tube in lab conditions given above (radius 2 m (6.6 ft)), the water would rise an unnoticeable 0.007 mm (0.00028 in). However, for a 4 cm (1.6 in) diameter tube (radius 2 cm (0.79 in)), the water would rise 0.7 mm (0.028 in), and for a 0.4 mm (0.016 in) diameter tube (radius 0.2 mm (0.0079 in)), the water would rise 70 mm (2.8 in).
Liquid transport in porous media
When a dry porous medium, such as a brick or a wick, is brought into contact with a liquid, it will start absorbing the liquid at a rate which decreases over time. For a bar of material with cross-sectional area A that is wetted on one end, the cumulative volume V of absorbed liquid after a time t is
where S is the sorptivity of the medium, with dimensions m/s1/2 or mm/min1/2. The quantity
is called the cumulative liquid intake, with the dimension of length. The wetted length of the bar, that is the distance between the wetted end of the bar and the so-called wet front, is dependent on the fraction f of the volume occupied by liquid. This number f is the porosity of the medium; the wetted length is then
Some authors use the quantity S/f as the sorptivity.[4] The above description is for the case where gravity and evaporation do not play a role.
Sorptivity is a relevant property of building materials, because it affects the amount of rising dampness. Some values for the sorptivity of building materials are in the table below.
| Material | Sorptivity (mm min-1/2) |
Source |
|---|---|---|
| Aerated concrete | 0.54 | [5] |
| Gypsum plaster | 3.50 | [5] |
| Clay brick | 1.16 | [5] |
Miscellaneous
Albert Einstein's first paper[6] submitted in 1900 to Annalen der Physik was on capillarity. It was titled Folgerungen aus den Capillaritätserscheinungen, which was translated as Conclusions from the capillarity phenomena, found in volume 4, page 513 (published in 1901).
See also
- Young–Laplace equation
- Capillary pressure
- Damp-proof course
- Capillary fringe
- Capillary wave
- Frost flowers
- Frost heaving
- Needle ice
- Washburn's equation
- Wick effect
- Bound water
- Surface Tension
References
- ^ http://science.jrank.org/pages/1182/Capillary-Action.html
- ^ http://en.wiktionary.org/wiki/capillary
- ^ G.K. Batchelor, 'An Introduction To Fluid Dynamics', Cambridge University Press (1967) ISBN 0-521-66396-2
- ^ C. Hall, W.D. Hoff, Water transport in brick, stone, and concrete. (2002) page 131 on Google books
- ^ a b c Hall and Hoff, p. 122
- ^ List of Scientific Publications of Albert Einstein